Background: Patients with relapsed or refractory (R/R) acute myeloid leukemia (AML) have dismal outcomes, representing an urgent unmet need. The BCL2 inhibitor venetoclax has exhibit encouraging anti-tumor activity in AML. The clinical efficacy of the CAG regimen in patients with R/R AML has been confirmed in previous studies. The aim of this study was to retrospectively compare the safety and efficacy of the combination of venetoclax with CAG regimen and CAG alone in patients with R/R AML.
Patients and methods: We retrospectively reviewed and compared the efficacy and safety of venetoclax combined with the CAG regimen (Ven-CAG group, n=37) and CAG regimen alone (CAG group, n=37) for 74 patients with R/R AML treated at our institution between August, 2016 and March 2023. The patients included those who were refractory to at least one cycle of induction chemotherapy, and patients with relapsed leukemia. According to the duration from complete remission (CR) to relapse, the relapsed patients were further divided into two subgroups, the favorable-risk relapsed group (the duration≥12 months) and the poor-risk relapsed group (the duration<12 months). The incidence of composite complete remission (CCR), minimal residual disease (MRD) status, one-year overall survival (OS), and adverse events (AEs) were analyzed and compared in two groups.
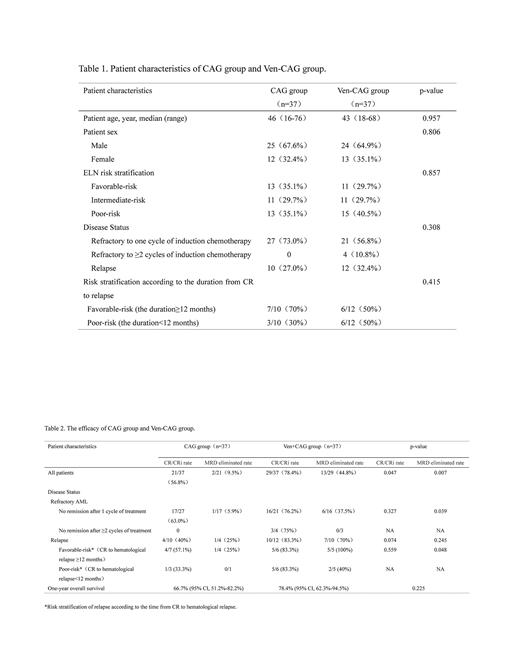
Results: Patient age, sex, ELN risk stratification, disease status, risk stratification according to the duration from CR to relapse were comparable between two groups (Table 1). The CCR of Ven-CAG group was 78.4% (29/37),which was superior to 56.8% (21/37) of CAG group (P=0.047) . Furthermore, the rate of MRD elimination in patients who achieved CCR in Ven-CAG group was significantly higher than that in CAG group [44.8% (13/29) vs. 9.5% (2/21), P= 0.007] (Table 2). The rate of MRD elimination in favorable-risk relapsed group (CR to hematological relapse ≥12 months) was higher in Ven-CAG group [100% (5/5) vs. 25% (1/4), P= 0.048]. The one-year OS were 78.4% (95% CI, 62.3%-94.5%) and 66.7% (95% CI, 51.2%-82.2%) in Ven-CAG and CAG group (P=0.225), respectively. The incidences of grade 3-4 hematologic AEs in Ven-CAG group and CAG group were 100% (37/37) and 97.3% (36/37), respectively (P=1.000). The incidences of febrile neutropenia were 67.6% (25/37) and 62.2% (23/37), respectively (P=0.626). Among patients who achieved CCR, the median time for neutrophil recovery in the Ven-CAG group and CAG group were 8 (3-30) days and 9.5 (3-31) days (P=0.781), while the median time for platelet recovery was 9 (3-28) days and 8 (3-19) days (P=0.708). The incidences of grade 3-4 non-hematologic AEs were comparable in Ven-CAG and CAG group (32.4% vs. 35.1%, P=0.806). The most common nonhematologic AEs were infections, with the incidences of 18.9% and 27%, respectively (P=0.407). No patients died within 4 weeks after initiating the induction course in Ven-CAG group. There was one case of early death during induction in CAG group due to cerebral hemorrhage.
Conclusions: Venetoclax added to CAG regimen was safe and active in patients with R/R AML, producing higher CCR and MRD-negative remissions than CAG regimen alone. These results highlight the incremental benefit of venetoclax added to CAG regimen. This requires further verification through prospective, larger size clinical trials.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal